BIO-3000-SB
UN 4GU/CLASS 6.2 (UN2814/UN2900) complete packaging
- ADR
- RID
- ADN
- IMDG
- IATA
UN 4GU/CLASS 6.2 (UN2814/UN2900) complete packaging
Packaging for Infectious Substances
*Supplied complete with: secondary container, cardboard insertion, 1 absorbent test-tube holder with 6 slots , 1 bubble-wrap pouch with 6 slots, LAB-T621-PC label (Infectious Substance), instruction sheet, security seals and outer cardboard packaging. Always available in stock packaging certified for the transport of Infectious Substance Category A. Infectious Substance Category A are divided into:
– UN 2814 INFECTIOUS SUBSTANCE AFFECTING HUMANS
– UN 2900 INFECTIOUS SUBSTANCE AFFECTING ANIMALS. The Infectious substances may, when exposure occurs, causing permanent disability or life-threatening or fatal disease to people or animals, which had been in good health.
For shipping Infectious Substance Category A (UN2814 – UN2900) you have to use a packaging certified for this substance. Our packagings are certified UN 4GU/Class 6.2 and in the rigid plastic secondary container can be inserted primary containers of any type (test tubes, blood vials, falcon, appendorf, swabs and sample containers).
This UN certified packaging can be used for testing Coronavirus swabs.
| Provided | Assembled with 95kPa tested secondary container and various products |
| In compliance with | P620 ADR, P620 RID, P620 IMDG, P.I. 602 IATA |
| Capacity | 0.95 L |
| Information | Compliant with the requests of the Ministry of Health for the transport of Coronavirus swabs (Circular no. 3 of 8 May 2003). |
| Country of origin | Italy |
| Dimensions | inner ø100 x 149 h mm |
| Dimensions | outer 130 x 130 x 180 h mm |
| Package | of 16 pieces |
| Content |
|---|
| Outer fibreboard packaging UN certified 4GU/Class 6.2, 95kPa tested from +40°C to +55°C secondary rigid plastic container, cardboard insertion, nr. 1 absorbent pouch with 6 slots, 1 bubble-wrap pouch with 6 slots, label LAB-T621-PC (Infectious Substance), instruction sheet, security seal. COVID-19 packaging suitable for shipping Coronavirus infected samples. |
| Neutral | |
| Certification | 95kPa from -40 °C to +55 °C |
|
Approval
(**Year of manufacture) |
4GU/CLASS.6.2/** B/SERPAC.403-090064 |
| Approved for |
|---|
| Primary container of all types (test-tubes, vials, flacon, appendorf) |
| Product | Material | Environmental code |
|---|---|---|
| Box | Corrugated cardboard |
PAP 20
|
| Insertion | Corrugated cardboard |
PAP 20

|
| Jar and cap | Polypropylene |
PP 5
|
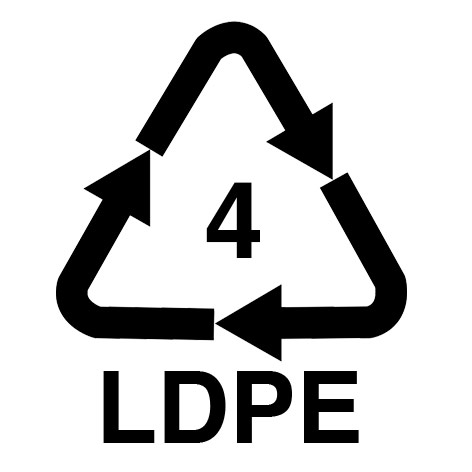
| Bubble wrap | PE (Polyethylene) |
LDPE 4
|
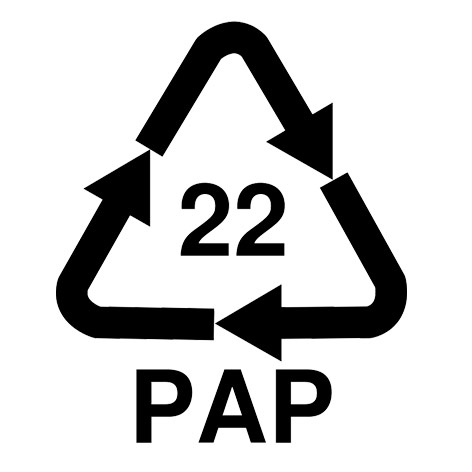
| Label | Paper |
PAP 22
|
| Instruction | Paper |
PAP 22

|
| Check your district's regulations. Separate the components and confer them properly. |
Click file link to download
(Some files may be visible and downloadable only after login)
UN 4GU/CLASS 6.2 (UN2814/UN2900) complete packaging
Packaging for Infectious Substances
*Supplied complete with: secondary container, cardboard insertion, 1 absorbent test-tube holder with 6 slots , 1 bubble-wrap pouch with 6 slots, LAB-T621-PC label (Infectious Substance), instruction sheet, security seals and outer cardboard packaging. Always available in stock packaging certified for the transport of Infectious Substance Category A. Infectious Substance Category A are divided into:
– UN 2814 INFECTIOUS SUBSTANCE AFFECTING HUMANS
– UN 2900 INFECTIOUS SUBSTANCE AFFECTING ANIMALS. The Infectious substances may, when exposure occurs, causing permanent disability or life-threatening or fatal disease to people or animals, which had been in good health.
For shipping Infectious Substance Category A (UN2814 – UN2900) you have to use a packaging certified for this substance. Our packagings are certified UN 4GU/Class 6.2 and in the rigid plastic secondary container can be inserted primary containers of any type (test tubes, blood vials, falcon, appendorf, swabs and sample containers).
This UN certified packaging can be used for testing Coronavirus swabs.
| Provided | Assembled with 95kPa tested secondary container and various products |
| In compliance with | P620 ADR, P620 RID, P620 IMDG, P.I. 602 IATA |
| Capacity | 0.95 L |
| Information | Compliant with the requests of the Ministry of Health for the transport of Coronavirus swabs (Circular no. 3 of 8 May 2003). |
| Country of origin | Italy |
| Dimensions | inner ø100 x 149 h mm |
| Dimensions | outer 130 x 130 x 180 h mm |
| Package | of 16 pieces |
| Content |
|---|
| Outer fibreboard packaging UN certified 4GU/Class 6.2, 95kPa tested from +40°C to +55°C secondary rigid plastic container, cardboard insertion, nr. 1 absorbent pouch with 6 slots, 1 bubble-wrap pouch with 6 slots, label LAB-T621-PC (Infectious Substance), instruction sheet, security seal. COVID-19 packaging suitable for shipping Coronavirus infected samples. |
| Neutral | |
| Certification | 95kPa from -40 °C to +55 °C |
|
Approval
(**Year of manufacture) |
4GU/CLASS.6.2/** B/SERPAC.403-090064 |
| Approved for |
|---|
| Primary container of all types (test-tubes, vials, flacon, appendorf) |
| Product | Material | Environmental code |
|---|---|---|
| Box | Corrugated cardboard |
PAP 20

|
| Insertion | Corrugated cardboard |
PAP 20

|
| Jar and cap | Polypropylene |
PP 5

|
| Bubble wrap | PE (Polyethylene) |
LDPE 4

|
| Label | Paper |
PAP 22

|
| Instruction | Paper |
PAP 22

|
| Check your district's regulations. Separate the components and confer them properly. |
Click file link to download
(Some files may be visible and downloadable only after login)